Project outline
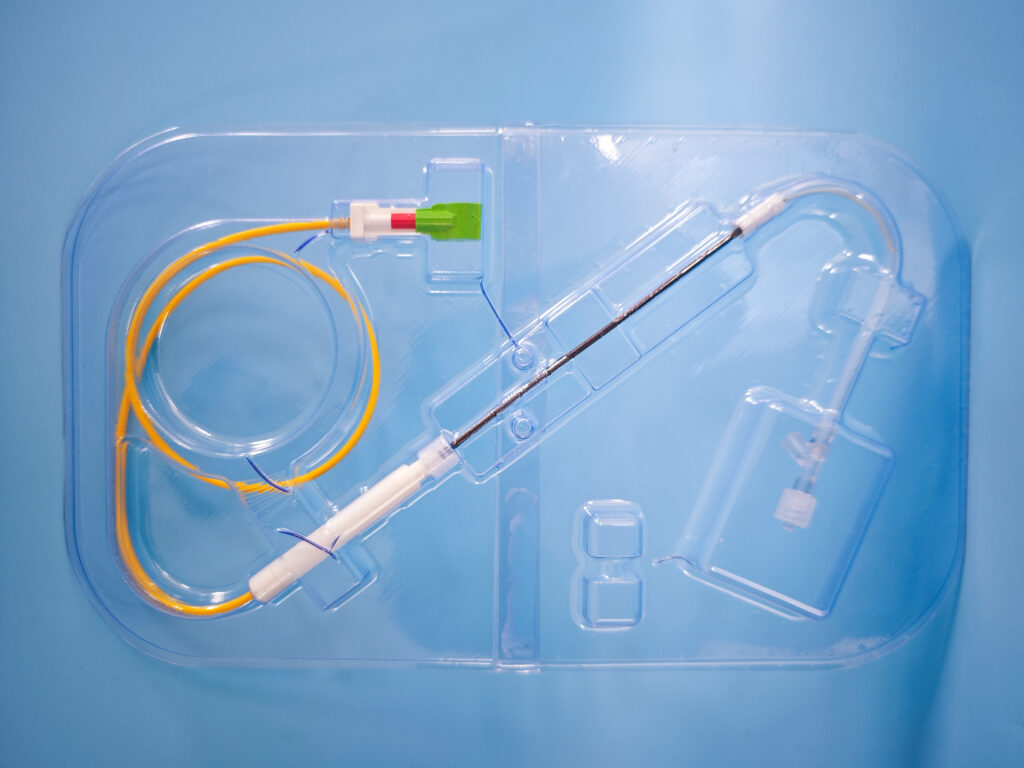
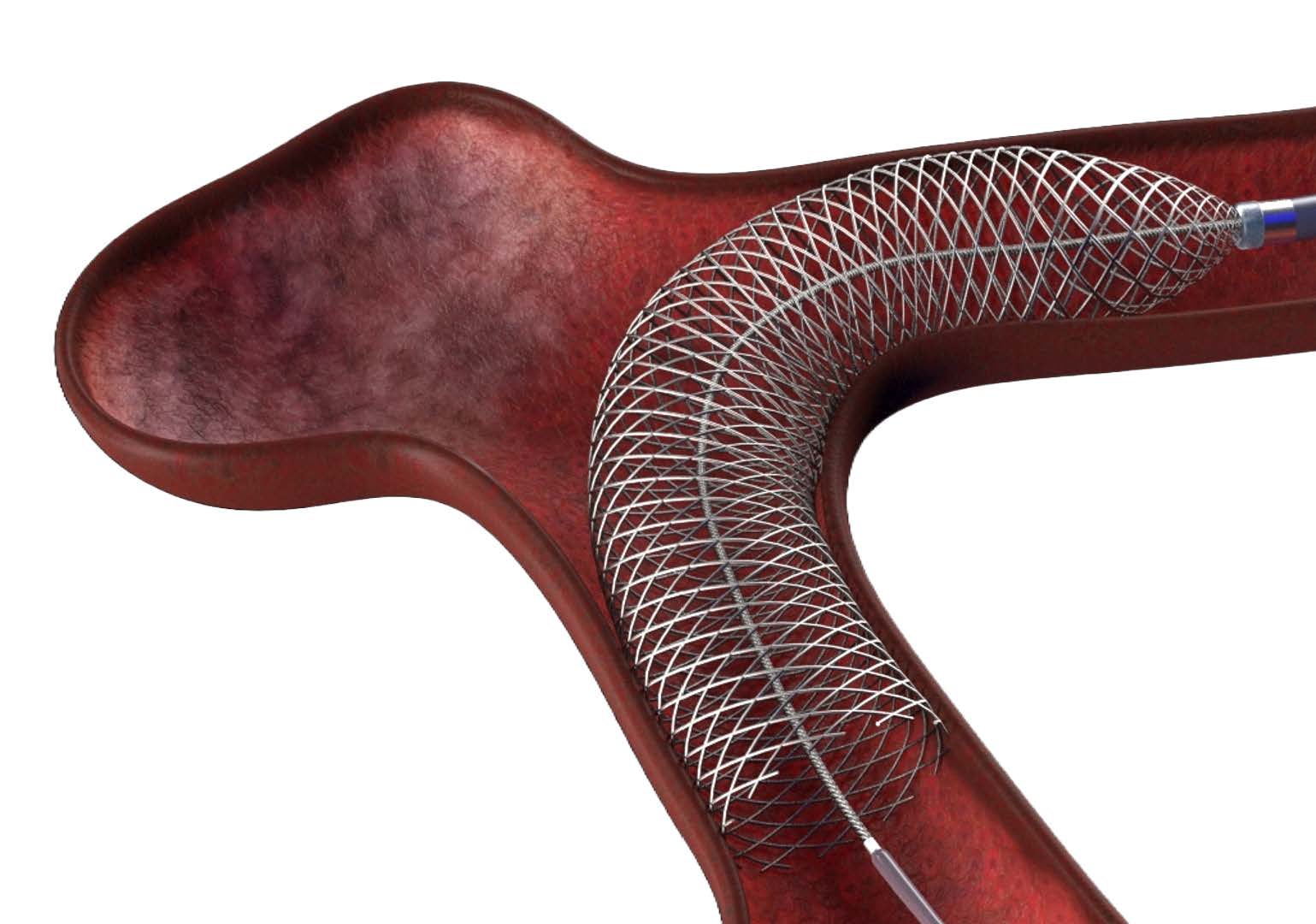
OxDevice was contracted to assemble braided and lasercut nitinol stents and load these onto delivery wires for a neurovascular application. The devices prevent brain aneurysms by diverting blood flow away from the aneurysm using a hybrid implant construction of a lasercut frame and braided nitinol stent, delivered and deployed by a delivery system.
Product development and process validation
The client produced in excess of 1000 detailed design documents that OxDevice translated into production specifications, including bespoke work instructions and process record cards to describe and record work undertaken during manufacture. OxDevice collaborated with the client closely in the development of a novel delivery system to deploy the device.
Our team of engineers produced custom-built equipment to assist in the manufacturing processes. OxDevice’s team of trained technicians assembled the braided and lasercut stent alongside the delivery system ready for Pre-V&V testing to verify the device’s design. This included goods inwards inspection of parts to 0.5 μm
resolution. Our engineering team assisted in the development of the device’s packaging including the pouching, boxing and labelling of the final product.
Dedicated risk management workshops were conducted by OxDevice’s engineers trained according to ISO 14971:2019, including process FMEAs for each of the manufacturing processes involved.