Selecting the ideal Manufacturing Partner
About
Our client, leaders in the field, needed a manufacturing partner with experience in the development and production of class 3 medical devices. Finding the contract manufacturer with the right skills and experience was crucial to the success of the project. OxDevice’s award winning team has a depth of experience in the design and production of class 3 implantable devices and soon became an extension of our client’s manufacturing department.

High Quality Product Assembly Assured
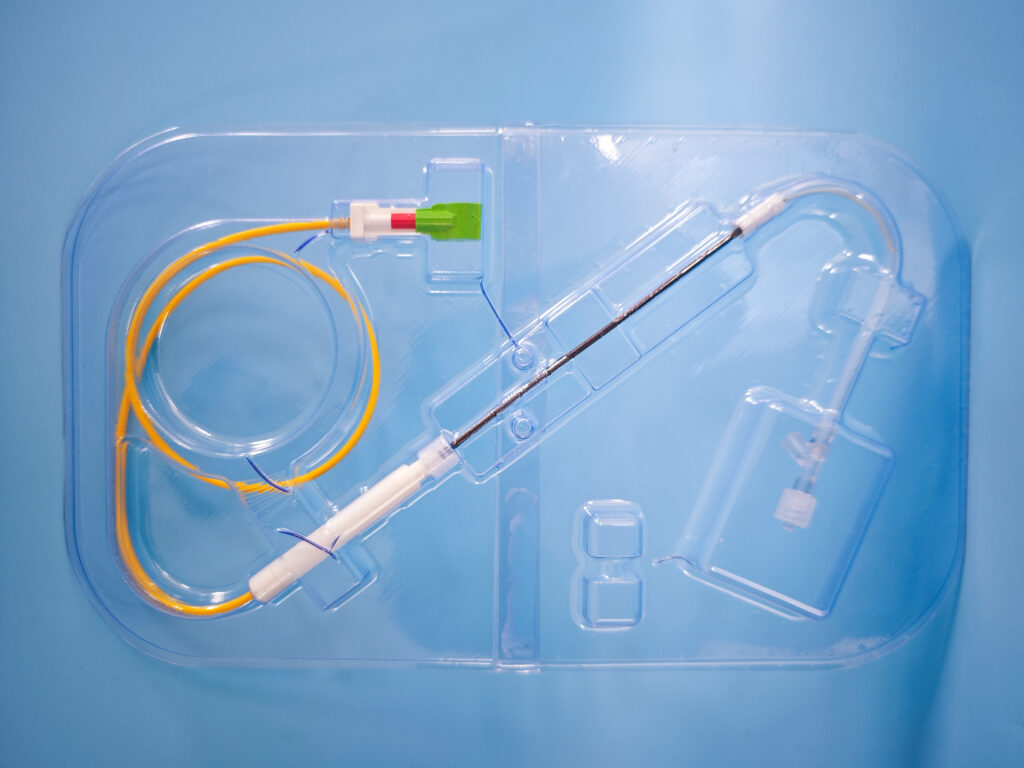
The assembly included both braided and lasercut nitinol stents loaded onto a nitinol delivery wire.
OxDevice ensured that the devices were produced to the highest standard of quality by developing production specifications detailing the inspection requirements for each of the assemblies, ensuring that any defects could easily removed and ensuring the product met our clients tolerances and requirements. OxDevice completed manufacture in our dedicated ISO Class 7 Cleanroom facility.
The Solution
The Project Manager at VascuShield had used OxDevice previously to help with the development of the initial concept of the device. He knew that he could get clinical and engineering expertise at a great price point. After bringing OxDevice in, the team quickly developed a realistic project plan to move the concept through to the pre-clinical stage.
Leveraging OxDevice’s expertise in endovascular implant and delivery system design and manufacturing enabled VascuShield to initially develop three concept directions. Each of these were prototyped in-house by OxDevice and trialled using state of the art pulse duplicator technology. Blood pressure and flow at different regions of the device was measured enabling comparisons with the advanced Computational Flow Dynamics performed by the VascuShield team. Laser cut nitinol stents were shape set and electropolished by OxDevice and assembled to complex ePTFE features.

Product Refinement
As part of the outsourcing contract, we worked with the client to develop the specification of the delivery wire, working as an extension of the client’s R&D department. Devices were manufactured to test the bond strength of the device, with testing undertaken and feedback given to the client resulting in an in specification bond strength delivery wire.
- Transfer to manufacturing – the client provided the drawings and OxDevice delivered the assemblies
OxDevice completed a Phase Review 4: Design Transfer to Design Review 4 standard to demonstrate the design intent had been correctly transferred into production specifications