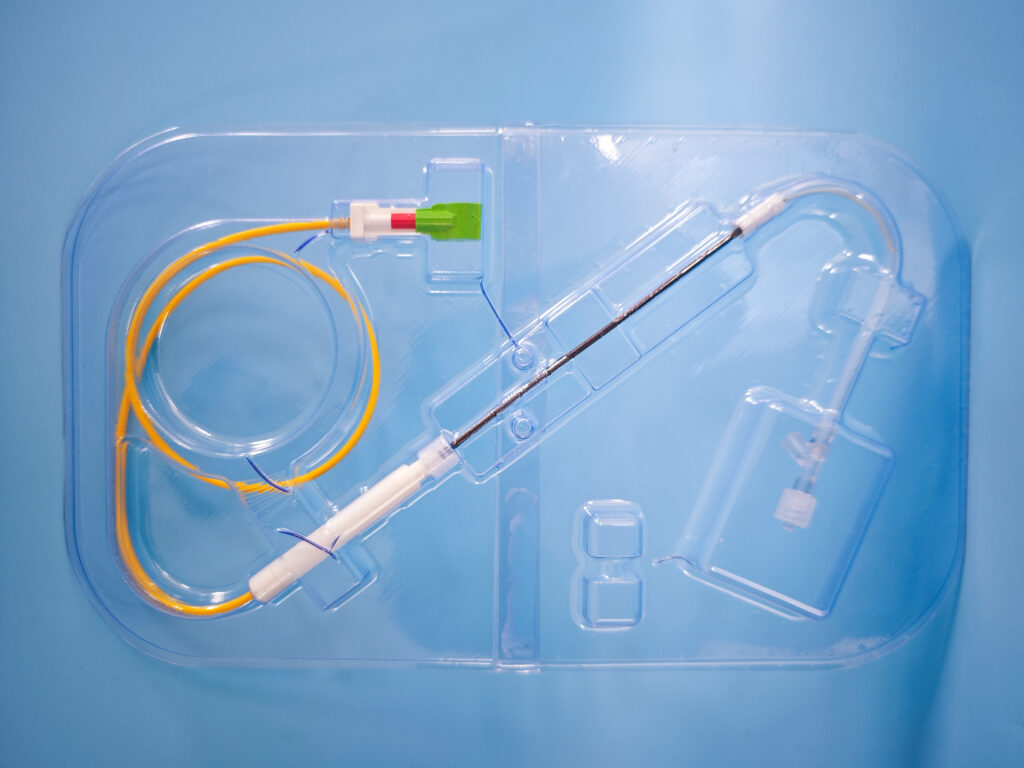
Client needs
OxDevice were contracted by a client to design and develop an implantable and removable embolic protection device ready for animal trial. The device aims to prevent strokes by deflecting blood clots away from the arteries leading to the brain from the Aorta.
Development Technologies & Techniques
OxDevice produced initial whiteboard concept designs and presented three concepts for Computer Aided Design in Solidworks. The design selected was a hybrid of braided and lasercut nitinol, to ensure optimal pore size whilst retaining position and flexibility once deployed.
OxDevice’s lasercut nitinol supplier produced the lasercut component, whilst the R&D team worked to produce the pictured prototypes on OxDevice’s in-house braiding rig.
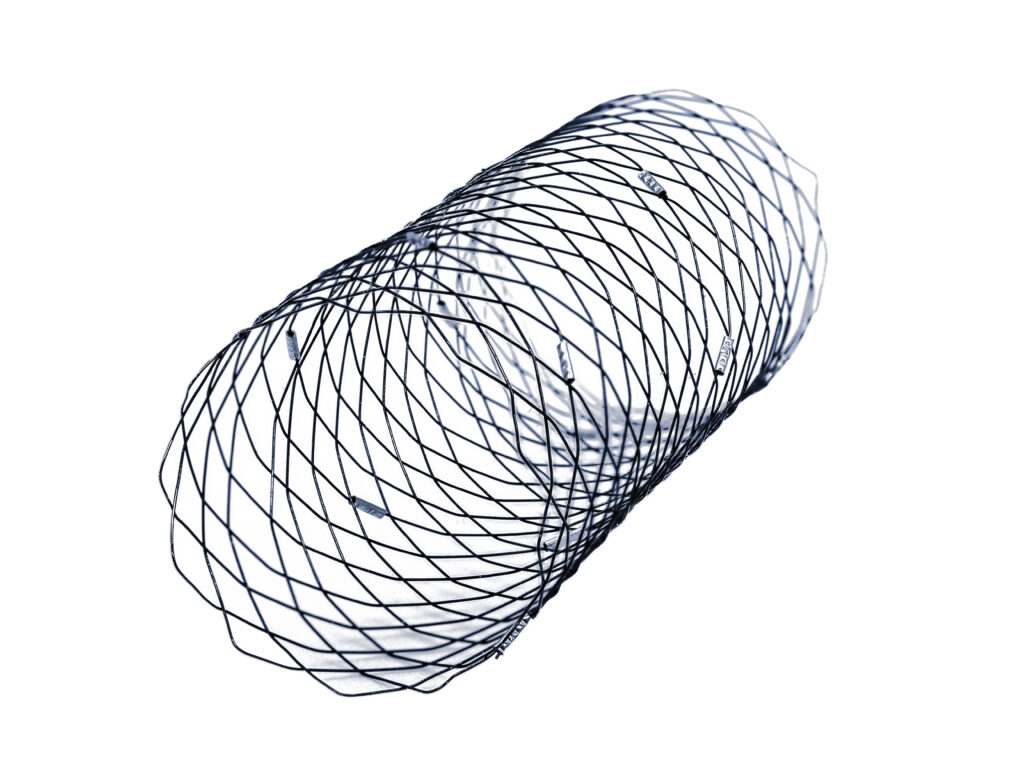
These samples were taken to our specialist nitinol heat treatment laboratory to shape set the prototypes to ensure they retain the desired shape upon deployment. The exact shape required was determined by conducting Anatomical analysis on client supplied patient CT scans.
Outcome
OxDevice successfully trained surgeons over skype on the use of the device, with all the devices deploying successfully as part of the animal trial, confirming the geometry of the design.
Product Refinement
As part of the outsourcing contract, we worked with the client to develop the specification of the delivery wire, working as an extension of the client’s R&D department. Devices were manufactured to test the bond strength of the device, with testing undertaken and feedback given to the client resulting in an in specification bond strength delivery wire.
- Transfer to manufacturing – the client provided the drawings and OxDevice delivered the assemblies
OxDevice completed a Phase Review 4: Design Transfer to Design Review 4 standard to demonstrate the design intent had been correctly transferred into production specifications