Project outline
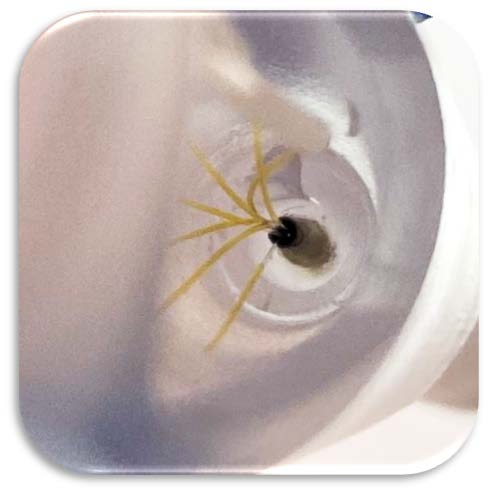
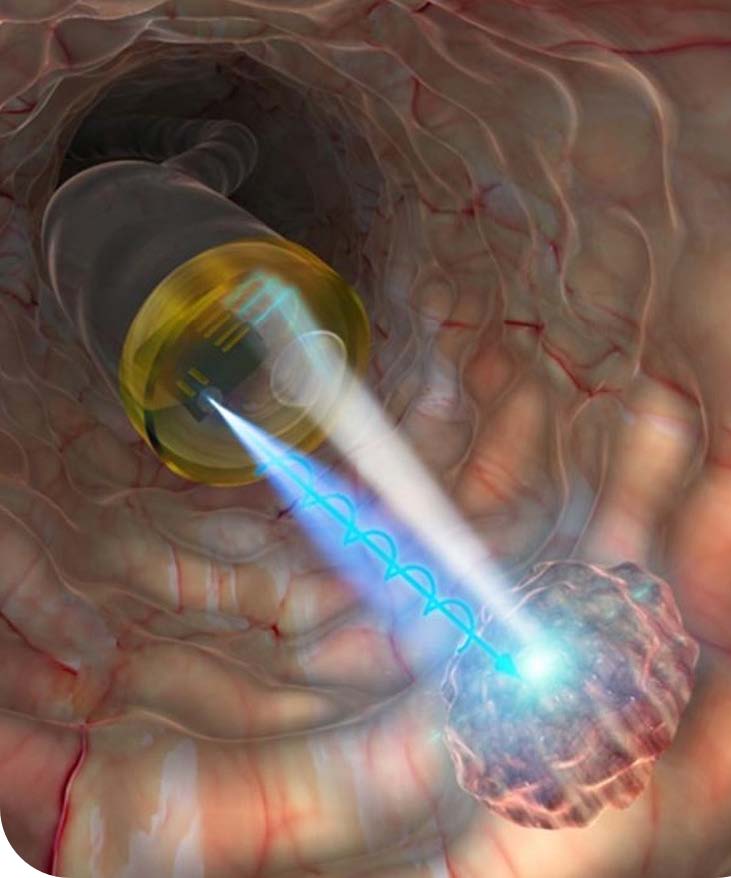
OxDevice was contracted to manufacture a fibre-optic enabled needle for the diagnosis of lymphoma in the neck glands. The remit of the project also included the design and development of the packaging for the device
Product development and process validation
The R&D team worked closely with our client to develop the specification of the parts of the device that were to be 3D printed on our dedicated biomedical resin tank. This close collaboration gave the client the confidence that the tolerances they had selected were correct prior to manufacture.
Our ISO 13485:2016 compliant QMS and experience in optical technologies enabled the design transfer of our customer’s design into manufacturing. In-house manufacturing processes include polishing and lapping of fibre optics, SLA 3D-printed handle housing assembly and needle assembly, completed under GMP conditions and process documentation.
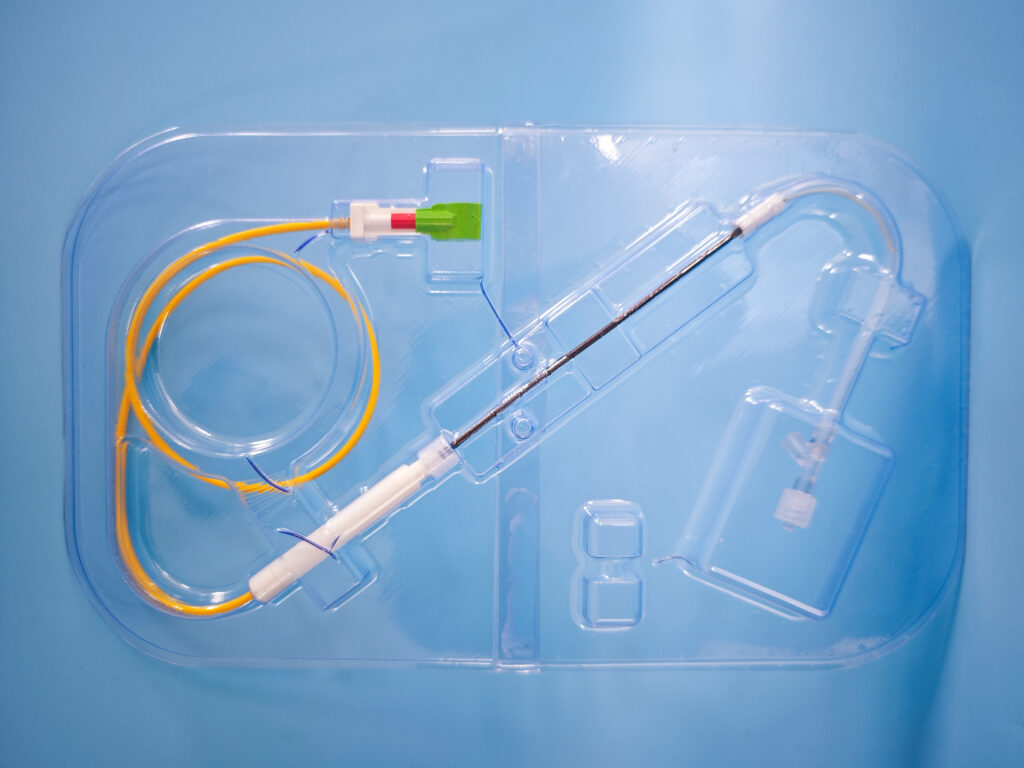
OxDevice provided onsite packaging validation facilities in accordance with BS EN ISO 11607-2 “Packaging for Terminally Sterilised Medical Devices” and EN 868-5:2018, to validate seals of pouches used as the sterile barrier of product prior to sterilisation. OxDevice developed and manufactured labelling and packaging for the devices, in accordance with ISO 15222-1:2021 Medical devices
Following the project delivery, the client has gained MHRA approval to start clinical trials.